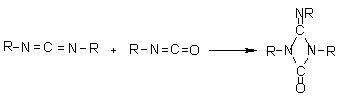1.2 - Isocyanates
Several isocyanate types
may be found in the market (Table 1.2).
Table 1.2 - Commercial Isocyanates
Common name /
CAS name |
Formula
|
Structure
|
Molecular Weight
|
Mp (°C)
|
Bp (°C)
|
Density (g/l)
|
nToxity
|
|
toluene 2,4-diisocyanate
(TDI) / 2,4-diisocyanato-1-mehyi-benzene
|
C9H6O2N2
|
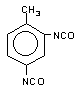
|
174,2
|
21,8
|
121 (10 mm Hg)
|
1,061 (20°C)
|
toxic
|
|
toluene 2,6-diisocyanate
(TDI) / 2,6-diisocyanato-1-mehyi-benzene
|
C9H6O2N2
|
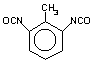
|
174,2
|
18,2
|
120 (10 mm Hg)
|
1,2271 (20°C)
|
toxic
|
|
65:35 mixture
of toluene 2,4 and 2,6-diisocyanate (TDI-65/35)
|
C9H6O2N2
|
|
174,2
|
5,0
|
121 (10 mm Hg)
|
1,222 (20°C)
|
toxic
|
|
80:20 mixture
of toluene 2,4 and 2,6-diisocyanate (TDI-80/20)
|
C9H6O2N2
|
|
174,2
|
13,6
|
121 (10 mm Hg)
|
1,221 (20°C)
|
toxic
|
|
4,4-diphenyl
methane diisocyanate (MDI ) / 1,1-methylenebis(4-isocyanato-benzene)
|
C15H10O2N2
|
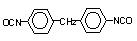
|
250,3
|
39,5
|
208 (10 mm Hg)
|
1,183 (50°C)
|
harmful to health
|
|
2,4-diphenyl
methane diisocyanate (MDI)/ 1-isocyanato-2-(4-isocyanatophenyl) methylbenzene
|
C15H10O2N2
|
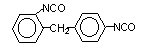
|
250,3
|
34,5
|
154 (1,3 mm Hg)
|
1,192 (40°C)
|
harmful to health
|
|
2,2-diphenyl
methane diisocyanate (MDI)/ 1,1-methylenebis(2-isocyanato-benzene)
|
C15H10O2N2
|
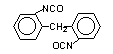
|
250,3
|
46,5
|
145 (1,3 mm Hg)
|
1,188 (50°C)
|
harmful to health
|
|
hexamethylene
diisocyanate (HDI) /1,6-diisocyanatohexane
|
C8H12O2N2
|
OCN-(CH2)6-NCO
|
168,2
|
-67
|
127 (10 mm Hg)
|
1,047 (20°C)
|
toxic
|
|
isophorone
diisocyanate (IPDI) / 5-isocyabato-1-(isocyanatomethy)-1,3,3-trimethylcyclohexane
|
C12H18O2N2
|
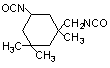
|
222,3
|
-60
|
158 (10 mm Hg)
|
1,061 (20°C)
|
toxic
|
|
m-tetramethylxylene
diisocyanate (m-TMXDI) / 1,3-bis(1-isocyanato-1-methylethy) benzene
|
C14H16N2O2
|
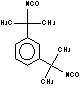
|
244,3
|
-
|
150 (50 mm Hg)
|
1,05 (20°C)
|
harmful to health
|
|
dicyclohexylmethane
4,4'-diisocyanate (HMDI) / 1,1-methylebis(4-isocyanato-cyclohexane)
|
C15H22O2N2
|
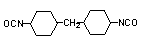
|
262,3
|
19-23
|
179 (10 mm Hg)
|
-
|
toxic
|
| triphenylmethane-4,4,4-triisocyanate
/ 1,1,1-methylidynetris (4-isocyanatobenzene) |
C22H13O3N3
|
|
367,4
|
91
|
-
|
-
|
harmful to health
|
|
naphthalene
1,5-diisocyanate (NDI) / 1,5- diisocyanatonaphthalene
|
C12H6O2N2
|
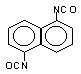
|
210,2
|
127
|
183 (10 mm Hg)
|
1,450 (20°C)
|
harmful to health
|
|
p-phenylene
diisocynate (PPDI) / 1,4-diidocyanatobenzene
|
C8H4O2N2
|

|
160,1
|
96
|
111 (12 mm Hg)
|
1,441 (20°C)
|
toxic
|
|
|
|
|
|
|
|
|
|
|
1.2.1
- Isocyanate Reactions
The manufacture of PU's
began as a chemists' empirical task, however the pioneers in this field tried
to explain the scientific secrets of this research area. Now, looking at the
past it is clear that many discoveries have been put forward since Otto Bayer's
original work. However there is still a lot of evidence of empirical character
in the chemistry of PU's. The observation of the isocyanate group electronic
structure indicates that the resonance structures in Figure 1.5 are possible.
The electron density is smaller in the carbon atom, average in nitrogen and
larger in oxygen. Isocyanate reactions mainly occur through addition to C=N
double bond. An active hydrogen atom-containing nucleophilic center attacks
the electrophilic carbon atom and active hydrogen is added to the nitrogen atom.
NCO-bonded electron acceptor groups increase the reactivity and donor groups
reduce it. For that reason, aromatic isocyanates are more reactive than aliphatic
ones. Reactivity is reduced by steric hindrance in isocyanate groups, and also
in active hydrogen compounds.
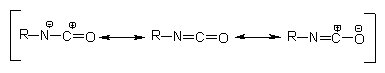
Figure 1.5 - Resonance structures of
the isocyanate group
The five main reactions
making up PU's technology are those of isocyanate with: (1) polyols, to form
polyurethanes; (2) amines, to yield polyurea; (3) water, to form polyurea and
carbon dioxide which is the main blowing agent in PU foams; (4) urethane and
(5) urea groups resulting in the formation of allophanate and biuret cross-linking,
respectively (Figure 1.6).

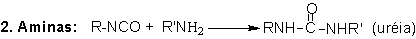
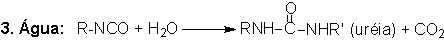
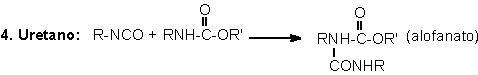
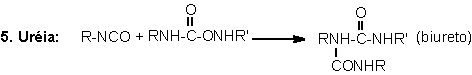
Figure 1.6 - Isocyanate
main reactions
1.2.1.1 - Reactions with alcohols
The
polymerization reaction (1), between an alcohol and an isocyanate is exothermic
and releases about 24 kcal/mol of urethane. Isocyanates reactivity with alcohols
is moderate (Table 2.2), being usually catalyzed by bases, mainly tertiary amines
or organometals. Reactivity is influenced by structure and primary, secondary
and tertiary hydroxyls have decreasing reactivity due to neighboring methyl
group steric hindrance. Amine basicity exerts a strong catalyst effect on isocyanate
reactions. Hydroxylated compounds with tertiary amino groups (like triethanolamine)
exhibit a catalytic effect.
1.2.1.2 - Reactions with amines
Reactions
(2) of isocyanates with amines, forming polyurea, are very fast (Table 1.3)
and don't require catalysis. Aliphatic amines react more quickly than aromatics
of lower basicity, since there isn't any significant steric hindrance. Aromatic
amines will be the less reactive as the larger is the electronegativity of the
aromatic ring substituents. In addition to electronic effects, the steric hindrances
are an important factor. The ortho position substituents of aromatic isocyanates
strongly reduce the reactivity. The highly reactive aliphatic amines are used
as chain extenders for polyurea, in reaction injection moulding (RIM) (Chapter
4) and in RIM-spray coatings (Chapter 7). On the other hand, the much less reactive
aromatic amines, as the methylene-bis-ortho-chloroaniline (MOCA) are used as
chain extenders for casting elastomers (Chapter 6).
1.2.1.3 - Reaction with water
The blowing reaction
(3), of isocyanates with water, results in urea formation and carbon dioxide
gas release. This reaction is very important in PU foam production. The carbon
dioxide diffusion to previously nucleated air bubbles blow the foam. The reaction
is exothermic and releases 47 kcal/mol of water. The isocyanate reactivity with
water and primary hydroxyl group is comparable, however much smaller than with
amines (Table 1.3). The catalysis of the blowing reaction is effected with tertiary
amines. Initially, carbamic acid is formed and then it decomposes into carbon
dioxide and the corresponding amine, which immediately reacts with diisocyanate,
yielding urea (Figure 1.7).
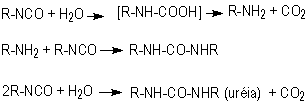
Figure 1.7 - Reaction
of isocyanate with water
1.2.1.4 - Reactions with ureas and urethanes
The hydrogen of urethane
and urea groups can react with NCO, forming allophanate and biuret cross-linking
groups. These reactions are reversible and occur at temperatures above 110oC,
and when not catalyzed, are slow and very slow, respectively (Table 1.3). They
mainly occur during the PU's post cure, where these are kept for a long time
at high temperatures (22 hours at 70°C), or days at room temperature, depending
on the system.
1.2.1.5 - Reactions with acids
Besides
the described five main reactions, isocyanates also react with carboxylic acids,
releasing carbon dioxide (Figure 1.8).
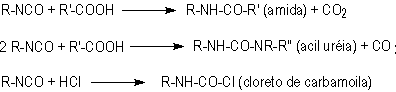
Figure 1.8 - Reaction
of isocyanates with acids
1.2.1.6 - Self-addition reactions
Isocyanates can also
react with themselves, forming dimers, trimers, polymers, carbodiimides and
uretoneimines (Figure 1.9). The dimerization of isocyanate to form uretidinediones
should be conducted at low temperatures in view of its thermal instability.
This explains why the isocyanates dimerization is limited to more reactive aromatic
ones. Isocyanates trimerization is of huge commercial importance, and with crude
MDI form polyisocyanurates used in rigid foams (Chapter 5). Carbodiimide formation
and further reaction with an excess isocyanate to form uretoneimines is also
of technical relevance for pure MDI modification, to form a liquid mixture of
melting point below 20°C.
| dimerization
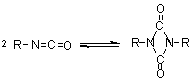 (uretidinedione) (uretidinedione)
|
|
trimerization
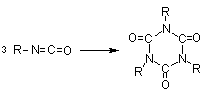 (isocyanurate) (isocyanurate)
|
|
carbodiimide
formation
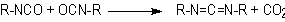
|
|
uretoneimine
formation
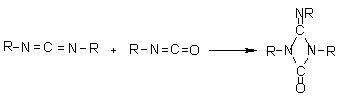
|
Figure 1.9 - Isocyanates self condensations
1.2.2 - Reactivity
The NCO group reactivity
depends on structure. The relative isocyanates reactivities with several active
hydrogen-containing compounds are shown in Table 1.3.
Table 1.3 - Relative reactivity of isocyanates
with active hydrogen compounds
|
Active hydrogen compound
|
Typical Structure
|
Relative reaction rate*
|
|
Primary
aliphatic amine
|
R-NH2
|
100.000
|
| Secondary
aliphatic amine |
RRNH
|
20.000 50.000
|
| Primary
aromatic amine |
Ar-NH2
|
200 300
|
| Primary
hydroxyl |
RCH2-OH
|
100
|
| Water |
HOH
|
100
|
| Carboxilic
acid |
RCOOH
|
40
|
| Secondary
hydroxyl |
RRCH-OH
|
30
|
| Ureas |
R-NH-CO-NH-R
|
15
|
| Tertiary
hydroxyl |
RRRC-OH
|
0,5
|
| Urethane |
R-NH-CO-O-R
|
0,3
|
| Amide |
RCO-NH2
|
0,1
|
*
uncatalyzed at 25oC
1.2.2.1 - Effect
of structure on reactivity
The isocyanate structure
is important for the NCO group reactivity. Reactivity is increased by substituents
that improve the positive load on the NCO group carbon atom. This is why aliphatic
isocyanates are less reactive than aromatic ones. These will be the more reactive
as the higher is the electro negativity of the aromatic ring substituent. In
addition to the electronic effect, steric hindrance is also important. Bulky
substituents near the reaction site reduce the reactivity. These steric factors
also influence the catalysts specificity, since catalysts need to be close to
the reaction site, to exert the desired catalytic effect, which may be impaired
by steric hindrance.
1.2.2.2 - Diisocyanates reactivity
Diisocyanate reactions are usually
more complicated than monoisocyanate reactions. The initial reactivity of an
aromatic diisocyanate is similar to that of a monoisocyanate with a NCO group
substituent. So when the first NCO reacts, for instance, with an alcohol, the
reactivity of the remainder NCO group is that of a monoisocyanate with an urethane
substituent group. As the urethane group is a much weaker activator than a NCO
group in the same position, in diisocyanates with both NCO groups in the same
aromatic ring the reactivity falls significantly when the reaction reaches 50%.
This decrease is still more accentuated if there is another substituent in the
ortho position relative to the second NCO, as in TDI.
In TDI the NCO group in the
para position reacts much more quickly than NCO in the ortho position. At room
temperature, if we consider as 100 the reactivity of the 2,4-TDI para NCO group,
the reactivity of the ortho NCO group would be 12. In 2,6-TDI the first NCO
group reactivity would be 56 and for the second group it would fall to 17. Though,
when temperature approaches 100oC, the steric effects are surpassed, and the
reactivity of both positions is similar. Due to this, the TDI type influences
some physical properties of foams. In diisocyanates with NCO groups in different
aromatic rings (like MDI), or separated by aliphatic chains, the reactivity
of NCO groups is the same.
1.2.3
- Commercial Isocyanates


![]()




![]()


![]()
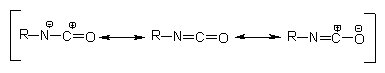
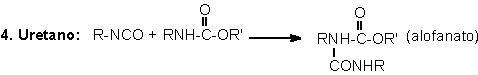
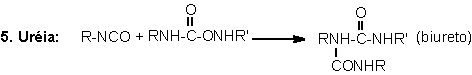
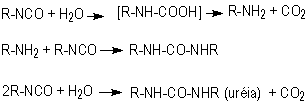
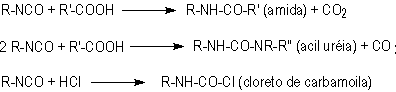
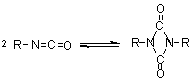 (uretidinedione)
(uretidinedione)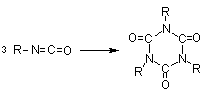 (isocyanurate)
(isocyanurate)